The indispensable safety feature for this hot air steriliser: Setpoint Wait. This means that the programme for sterilisation only starts when the set temperature has been reached. This feature can also be used with freely positionable Pt100 temperature sensors. Here the sterilisation time only begins when the set temperature has been reached at all measurement points, and reliable sterilisation is guaranteed at all times.
On this page, you can find all the essential technical data on the Memmert hot air steriliser. Our customer relations team will be pleased to help if you want further information. If you should require a customised special solution, please contact our technical specialists at sales@memmert.com.
Temperature
| Setting temperature range | +20 to +250 °C |
|---|---|
| Setting accuracy temperature | up to 99.9 °C: 0.1 / from 100 °C: 0.5 |
| Working temperature range | at least 5 above ambient temperature to +250 °C |
| Temperature sensor | 1 Pt100 sensor DIN class A in 4-wire-circuit |
Control technology
| ControlCOCKPIT | SingleDISPLAY. Adaptive multifunctional digital PID-microprocessor controller with high-definition TFT-colour display |
|---|---|
| Timer | Digital backwards counter with target time setting, adjustable from 1 minute to 99 days |
| Function SetpointWAIT | the process time does not start until the set temperature is reached |
| Calibration | three freely selectable temperature values |
| adjustable parameters | temperature (Celsius or Fahrenheit), air flap position, programme time, time zones, summertime/wintertime |
Ventilation
| Convection | natural convection |
|---|---|
| Fresh air | Admixture of pre-heated fresh air by electronically adjustable air flap |
| Vent | vent connection with restrictor flap |
Communication
| Documentation | programme stored in case of power failure |
|---|---|
| Programming | AtmoCONTROL software for reading out, managing and organising the data logger via Ethernet interface (temporary trial version can be downloaded). USB stick with AtmoCONTROL software available as accessory (on demand). |
Safety
| Temperature control | adjustable electronic overtemperature monitor and mechanical temperature limiter TB, protection class 1 according to DIN 12880 to switch off the heating approx. 20°C above nominal temperature |
|---|---|
| Autodiagnostic system | for fault analysis |
Standard equipment
| Works calibration certificate | Calibration at +160°C |
|---|---|
| Door | fully insulated stainless steel door with 2-point locking (compression door lock) |
| Internals | 2 stainless steel grid(s), electropolished |
Stainless steel interior
| Interior | easy-to-clean interior,made of stainless steel, reinforced by deep drawn ribbing with integrated and protected large-area heating on four sides |
|---|---|
| Volume | 108 l |
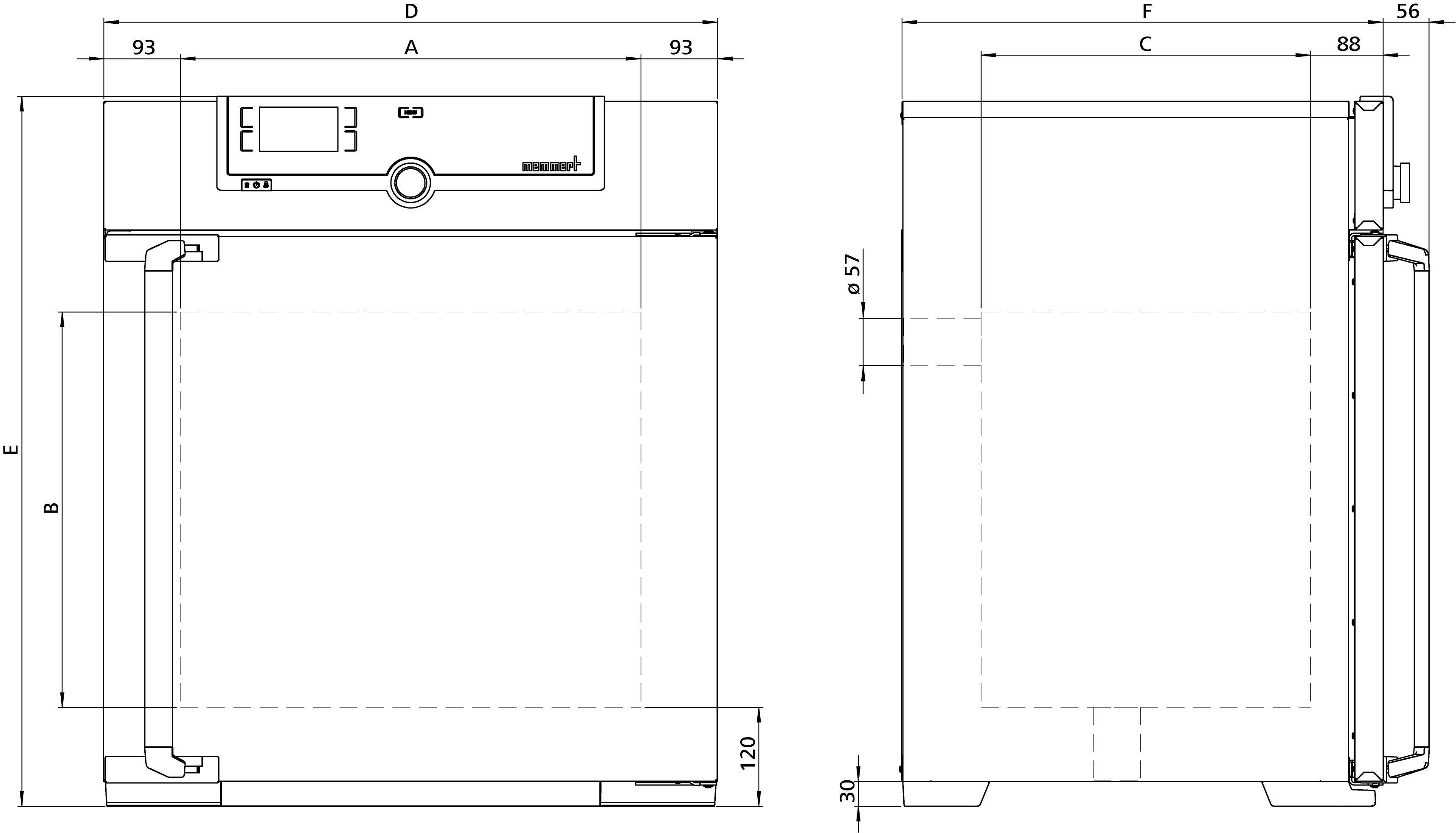
| Dimensions | w(A) x h(B) x d(C): 560 x 480 x 400 mm |
| Max. number of internals | 5 |
| Max. loading of chamber | 175 kg |
| Max. loading per internal | 20 kg |
Textured stainless steel casing
| Dimensions | w(D) x h(E) x d(F): 745 x 864 x 584 mm (d +56mm door handle) |
|---|---|
| Housing | rear zinc-plated steel |
Electrical data
| Voltage Electrical load | 230 V, 50/60 Hz approx. 2800 W |
|---|---|
| Voltage Electrical load | 115 V, 50/60 Hz approx. 1800 W |
Ambient conditions
| Set Up | The distance between the wall and the rear of the appliance must be at least 15 cm. The clearance from the ceiling must not be less than 20 cm and the side clearance from walls or nearby appliances must not be less than 5 cm. |
|---|---|
| Altitude of installation | max. 2,000 m above sea level |
| Ambient temperature | +5 °C to +40 °C |
| Humidity rh | max. 80 %, non-condensing |
| Overvoltage category | II |
| Pollution degree | 2 |
Packing/shipping data
| Transport information | The appliances must be transported upright |
|---|---|
| Customs tariff number | 8419 8998 |
| Country of origin | Federal Republic of Germany |
| WEEE-Reg.-No. | DE 66812464 |
| Dimensions approx incl. carton | w x h x d: 830 x 1050 x 800 mm |
| Net weight | approx. 74 kg |
| Gross weight carton | approx. 99 kg |
Standard units are safety-approved and bear the test marks




Intended use as medical device
Memmert is bringing medical devices of class I according to EU Directive (EU) 2017/745 into circulation.
| Type | Intended use as medical device | Classification according to (EU) 2017/745 |
|---|---|---|
| UNmplus, UFmplus | The appliance may be used for heating fango, silicate and APS packs for physical therapy and keeping them warm. | I |
| UNm, UFm, INm, IFm | The appliance may be used for heating fango, silicate and APS packs for physical therapy and keeping them warm. | I |
| INmplus, IFmplus | Implus may be used for temperature control of rinsing and infusion solutions and contrast agents. | I |
| IFbw | The appliance is used to heat non-sterile cloths and covers. Any other use is improper and may cause damage or danger. |
I |
Memmert is bringing medical devices of class IIa and IIb into circulation according to MDD 93/42/EEC until 31.12.2028 according to the transitional provisions (EU) 2023/607 by the (EU) 2017/745 article 120 (2).
| Type | Intended use as medical device | Classification according to 93/42/EWG |
|---|---|---|
| SNplus, SFplus | The product is intended for the sterilisation of medical devices with dry heat at atmospheric pressure. | IIb |
| SN, SF | The product is intended for the sterilisation of medical devices with dry heat at atmospheric pressure. | IIb |
| ICOmed | The CO2 incubator ICOmed is used to generate and maintain constant ambient conditions for the in-vitro fertilisation (IVF) application field, especially for the incubation of oocytes, spermatozoa and zygotes in special culture dishes for IVF application as well as for gene expression and the biosynthesis of RNA and proteins. | IIa |